Product Description
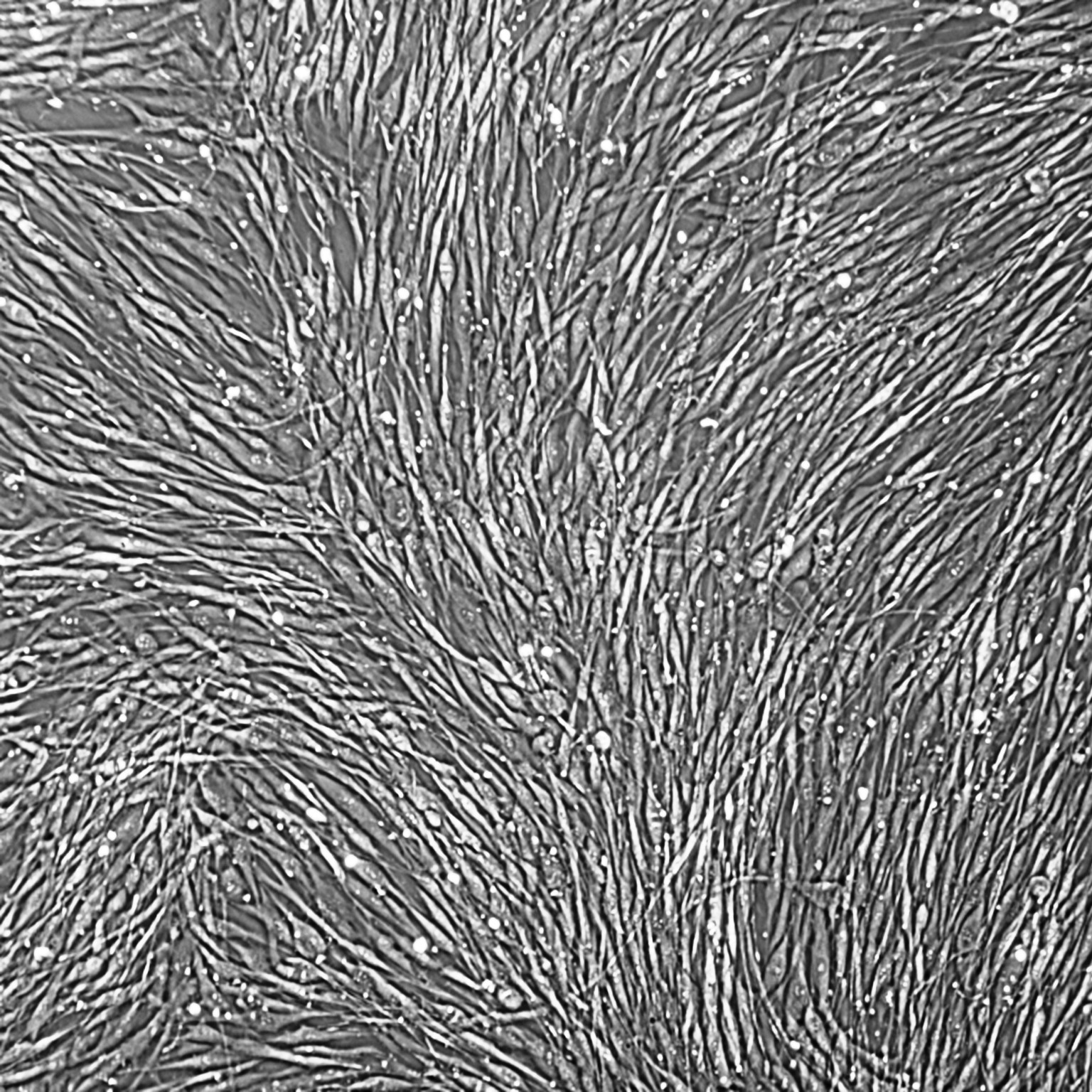
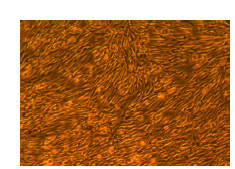
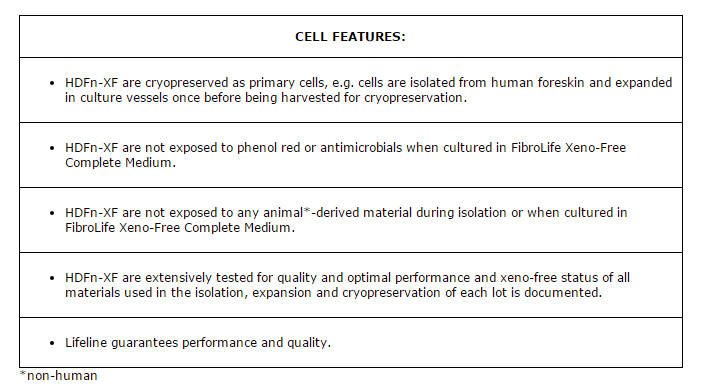
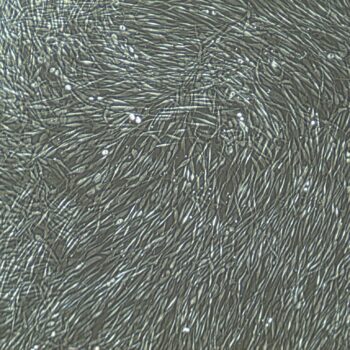
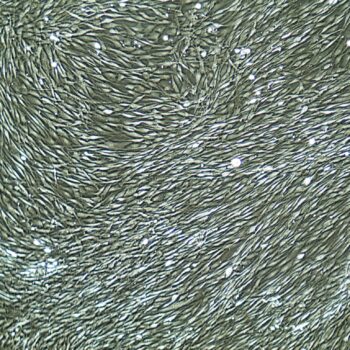
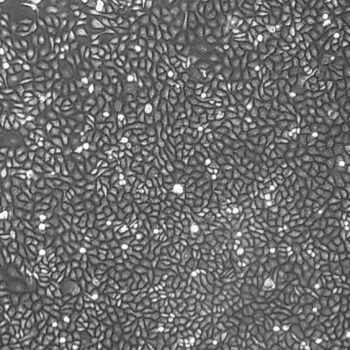
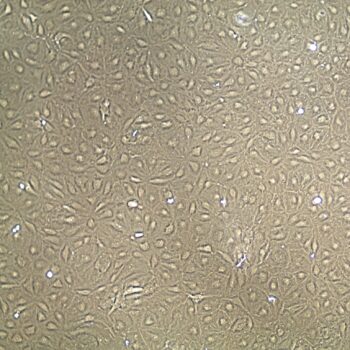
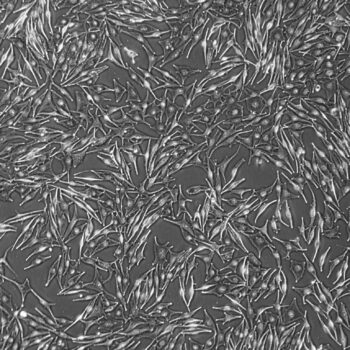
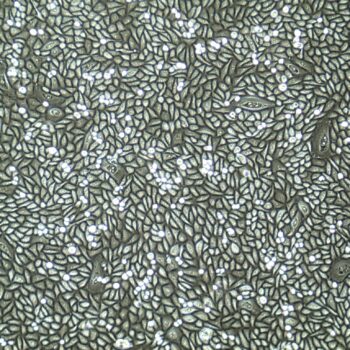
Lifeline® Primary Normal Human Dermal Fibroblasts-Neonatal, Xeno-Free (HDFn-XF), when grown in FibroLife® Xeno-Free Complete Medium, provide an ideal xeno-free culture system to establish xeno-free human feeder layers for human pluripotent stem cell cultures or as a model to study wound healing, toxicology or basic cell biology. They have not been exposed to any non-human or animal material.
- Primary Neonatal Skin Fibroblasts are directly cultured from their source organ tissue and have not been modified in any way. This helps avoid altering the outcome of your experiments.
- Your research outcomes will be more representative of the general population: Primary Neonatal Skin Fibroblasts can come from many donors.
Primary Neonatal Dermal Fibroblasts are cryopreserved after primary culture to ensure the highest viability and plating efficiency. Our HDFn-XF are quality tested in FibroLife Xeno-Free Complete Medium to ensure optimal growth over a period of at least 15 population doublings at rates equal to or greater than other commercially available media containing non-human proteins or supplements.
Lifeline Primary Fibroblasts are not exposed to antimicrobials or phenol red when cultured in FibroLife Xeno-Free Complete Medium, an advantage since these supplements can cause cell stress and ” masking effects” that may negatively impact experimental results.
500,000 cells per vial
Neonatal dermal fibroblasts are tested for:
- Proliferation and morphology: Normal cell appearance for 15 population doublings
- Cell viability: Minimum 70% viability when thawed from cryopreservation
- Sterility: Negative for mycoplasma. Negative for bacterial and fungal growth
- Virus: Negative for HIV-1, HIV-2, HBV, and HCV by PCR
Lifeline documents that all materials used in the manufacture of products which are labeled “Xeno-Free” have never come into contact with material of animal (non-human) origin and are not of animal (non-human) origin.
Product Specification Sheets
Fibroblast Systems
Skin Cell Systems
Complementary Products
SDS and “Safety Data Sheets”
(MSDS or Material Safety Data Sheets)
Cells
Protocols & Instructions
- Differentiating Fibroblasts to iPS
- Normal Human Dermal Fibroblasts-Neonatal, Xeno-Free (HDFn-XF)
- Normal Human Fibroblasts
FC-0037