iPS Validated Fibroblast Cell Culture Systems
Lifeline® Normal Human Dermal Fibroblasts have been validated for their ability to become Induced Pluripotent Stem Cells (iPS). Neonatal Fibroblast cells, LOT#00866, can be transduced with lentiviral vectors with very high efficiencies. Normal Human Dermal Fibroblasts derived from neonatal skin are cryopreserved as primary cells to ensure the highest purity, viability, and plating efficiency. These Fibroblasts are quality tested in FibroLife® Serum-Free Medium and demonstrate optimal growth at rates equal to or greater than classical serum-supplemented media.
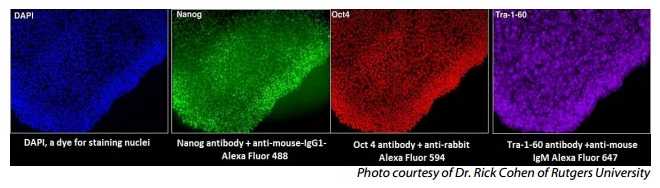
Lifeline® Xeno-Free Normal Human Dermal Fibroblasts are quality tested in FibroLife® XenoFree Medium which supports fibroblast growth, without the use of animal-derived components, at rates equal to or greater than media supplemented with 2-10% fetal bovine serum. Transduction of Normal Human Dermal Fibroblast LOT#00866 with lentiviral vectors and subsequent fluorescent labeling with antibodies to Nanog, Oct 4, and Tra-1-60 demonstrates very high levels of expression for these biomarkers of Pluripotency and iPS cells.
Quality Testing for Guaranteed Consistency and Reproducible Results
Lifeline® Cell Technology manufactures products using the highest quality raw materials and incorporates extensive quality assurance in every production run. Exacting standards and production procedures ensure consistent performance.
Please, contact your Lifeline® Account Representative to purchase iPS Validated Fibroblasts or for more information.