FrostaLife ™ Cryopreservation Solution — 100 mL
$188.70
In stock
Product Description
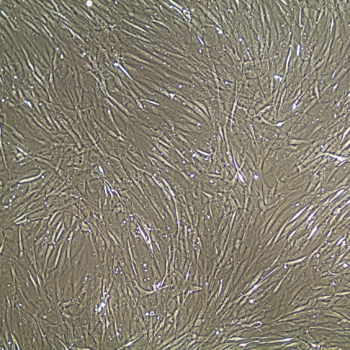
FrostaLife™ is a solution optimized for the cryopreservation of Primary Mammalian Cells by Lifeline® cell culture experts. Simply count your cells, centrifuge them and resuspend the cell pellet in the desired volume of FrostaLife and dispense into cryopreservation vials.
FrostaLife is a ready-to-use 100 mL freezing reagent containing DMSO and fetal bovine serum. FrostaLife contains no antimicrobials and no phenol red.
Quality Testing for Guaranteed Consistency and Reproducible Results
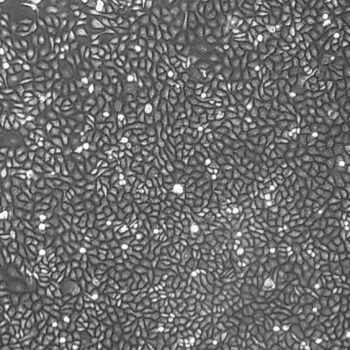
Lifeline Cell Technology manufactures our cell freezing solutions using the highest quality raw materials and incorporates ISO- style quality assurance in every production run. Exacting standards and production procedures ensure lot-to-lot consistency. FrostaLife cryopreservation solution is extensively tested using Primary Human Melanocytes.
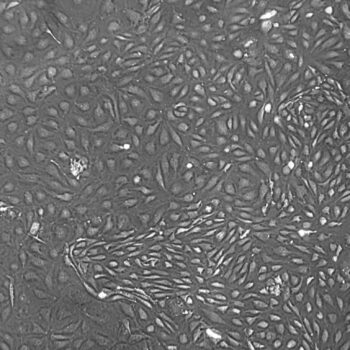
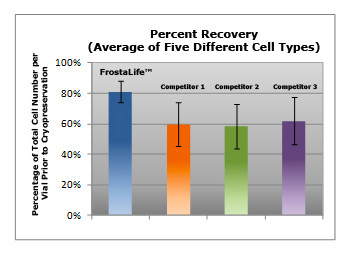 In comparisons with other commercially-available cryopreservation solutions, FrostaLife shows better post-thaw viability and cell reattachment, and better overall recovery (fewer lysed/destroyed cells).
In comparisons with other commercially-available cryopreservation solutions, FrostaLife shows better post-thaw viability and cell reattachment, and better overall recovery (fewer lysed/destroyed cells).
Shown left: Average recovery of cells after cryopreservation: Primary Human Epidermal Keratinocytes, Primary Dermal Microvascular Endothelial Cells, Human Mesenchymal Stem Cells, Primary Human Epidermal Melanocytes, and Primary Human Umbilical Vein Endothelial Cells were cultured and cryopreserved in various cryopreservation solutions. After thawing the vials, cells were counted and the total number of cells (viable and non-viable) was compared to the total number of viable cells that were dispensed prior to cryopreservation.
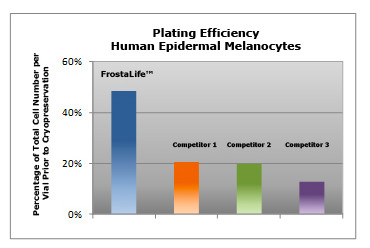
Shown above: Post-thaw plating efficiency: Primary Human Epidermal Melanocytes, were cultured in DermaLife M Melanocyte Medium and cryopreserved in various cryopreservation solutions. After thawing the vials, cells were counted and inoculated into flasks. The following day, the cells that had attached were removed from the flask and counted. This value was compared to the total number of viable cells inoculated.
Product Specification Sheets
Fibroblast Systems
Skin Cell Systems
Male Reproductive Cell Systems
Stem Cell Systems
Cryopreservation Solutions
Complimentary Products
- Human Aortic Smooth Muscle Cells [FC-0015]
- Human Renal Medullary Epithelial Cells, Primary [FC-0018]
- Normal Human Aortic Endothelial Cells [FC-0014]
- Normal Human Renal Mixed Epithelial Cells, Primary [FC-0017]
- Normal Human Small Airway Epithelial Cells, Primary [FC-0016]
- Epidermal Keratinocytes — Neonatal, Primary [FC-0007]
- Human Dermal Fibroblasts – Neonatal, Primary [FC-0001]
- Human Renal Cortical Epithelial Cells, Primary [FC-0012]
- Normal Human Umbilical Vein Endothelial Cells (HUVEC), Primary [FC-0003]
- Renal Proximal Tubule Epithelial Cells [FC-0013]
SDS and “Safety Data Sheets”
(MSDS or Material Safety Data Sheets)
Media
Product Specification Sheets
Mesenchymal Stem Cells - Human
Reproductive Cell Systems
FrostaLife™
Renal Epithelial Cells
Stem Cell Systems
Smooth Muscle Cell Systems
Prostate Epithelial cells (HPrEC) - Human
Endothelial Cells - Human
Mammary Epithelial Cells
Male Reproductive Cell Systems
Female Reproductive Cell Systems
Endothelial Cell Systems
Normal Human Epidermal Melanocytes-Neonatal (HEMn)
Fibroblast Systems
Oral Keratinocytes - Human
Skeletal Muscle - Human
Epidermal Keratinocytes - Human
Cervical Epithelial Cells
Corneal Epithelial Cell Systems
Bladder Epithelial Cells - Human
Airway Epithelial Cells - Human
Normal Human Airway Epithelial Cells